24
lipid compositions tested computationally
3
compositions selected
for validation
x20
drug load increase
achieved
Stability range
of
nanoparticle formulations
assessed
01/ Background
- Two IND-stage drug candidates act simultaneously as
agonists of toll-like receptors TLR4 and TLR7. - The compounds are intended to be delivered in liposomes or
lipidic nanoparticles, but the drug load was insufficient. - The goal was to perform the computational search for
optimal lipid composition for both formulations.
02/ Methodology
- 24 different lipid compositions were tested computationally using
a combination of molecular dynamics techniques:
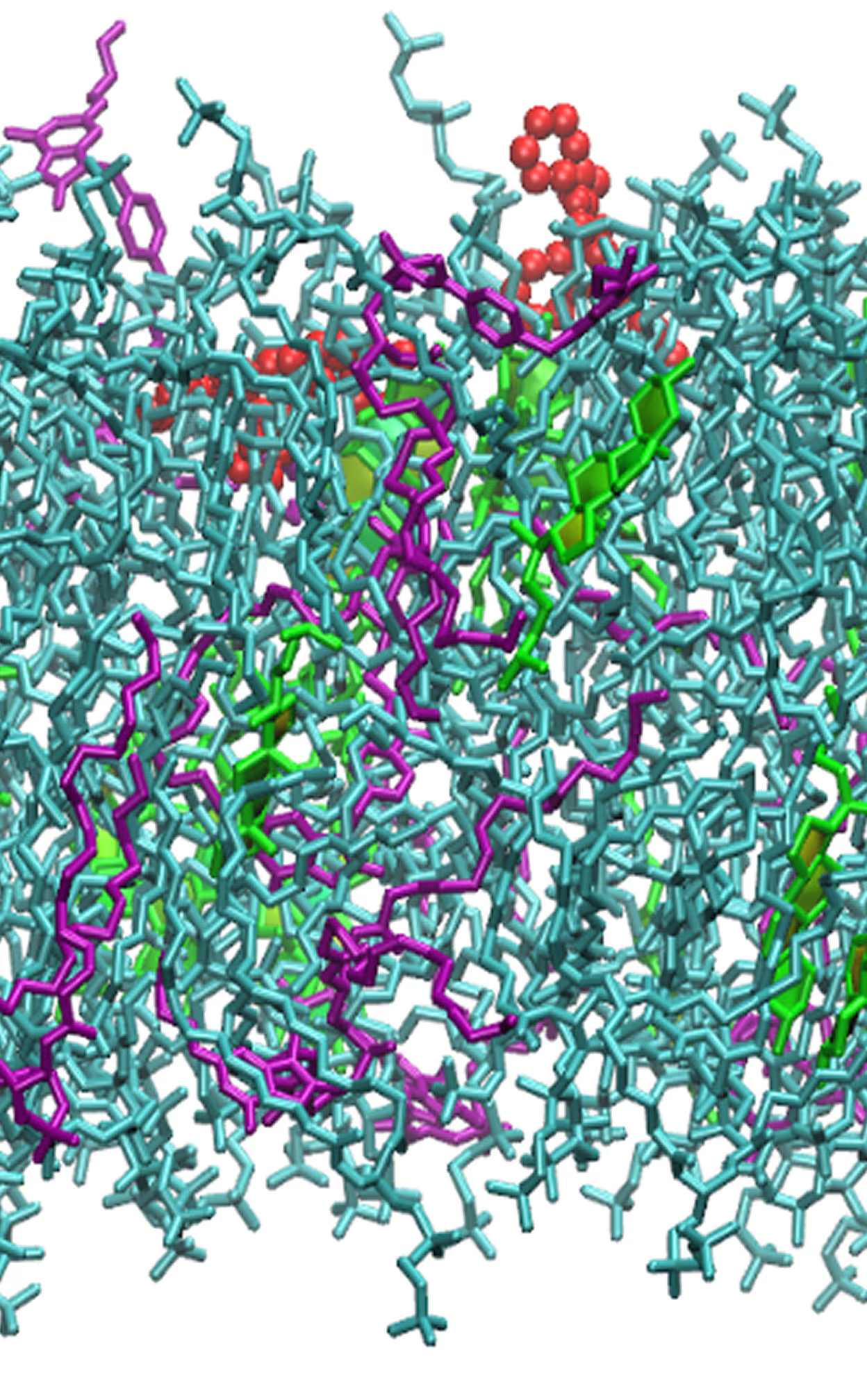
- Spontaneous incorporation into the bilayers.
- Alchemical free energy simulations of incorporation.
- Self-assembly of drug-lipid mixtures.
- Umbrella sampling simulation of drug incorporation.
- Self-assembly of nanoparticles formed by pristine drugs and their mixtures was simulated under different conditions.
03/ Liposome Formulation Results
- 3 compositions maximizing the free energy of drug incorporation into the lipid membrane selected.
- Experimental validation confirmed these 3 compositions
increase drug load up to 20 times relative to initial composition.
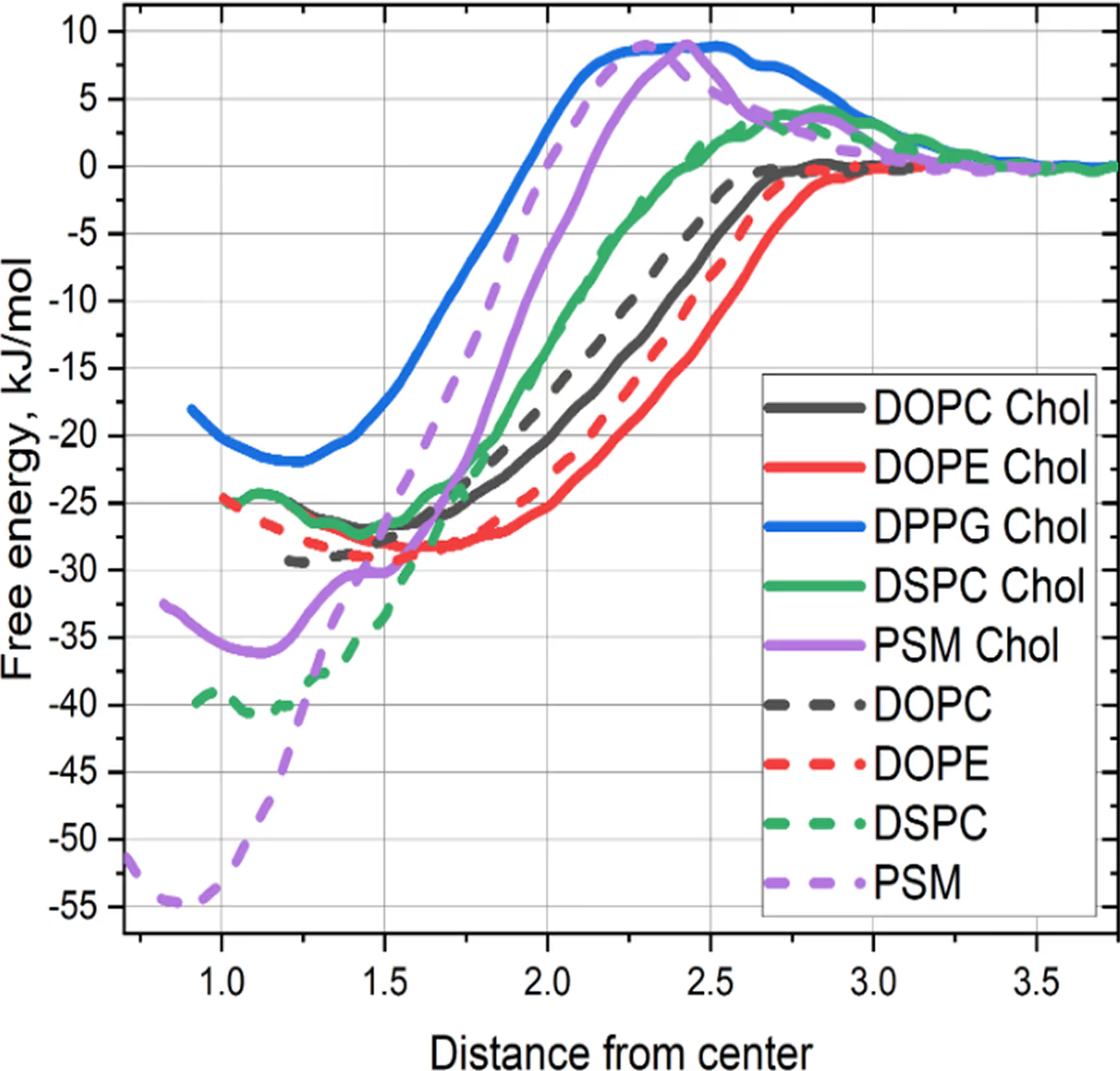
*Potentials of mean force for drug incorporation into the bilayers of different composition
(the lower the minimum — the better)
(the lower the minimum — the better)
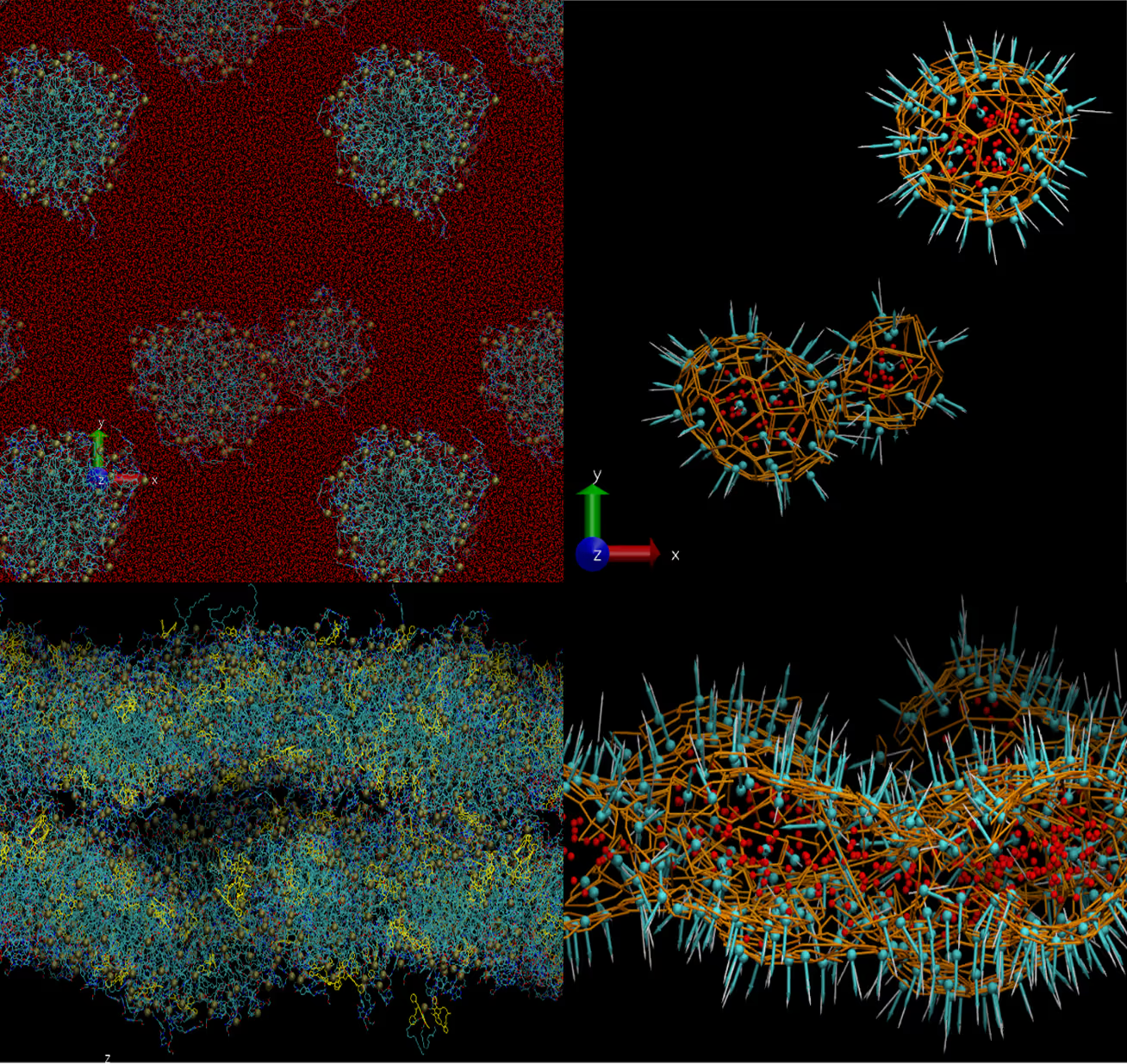
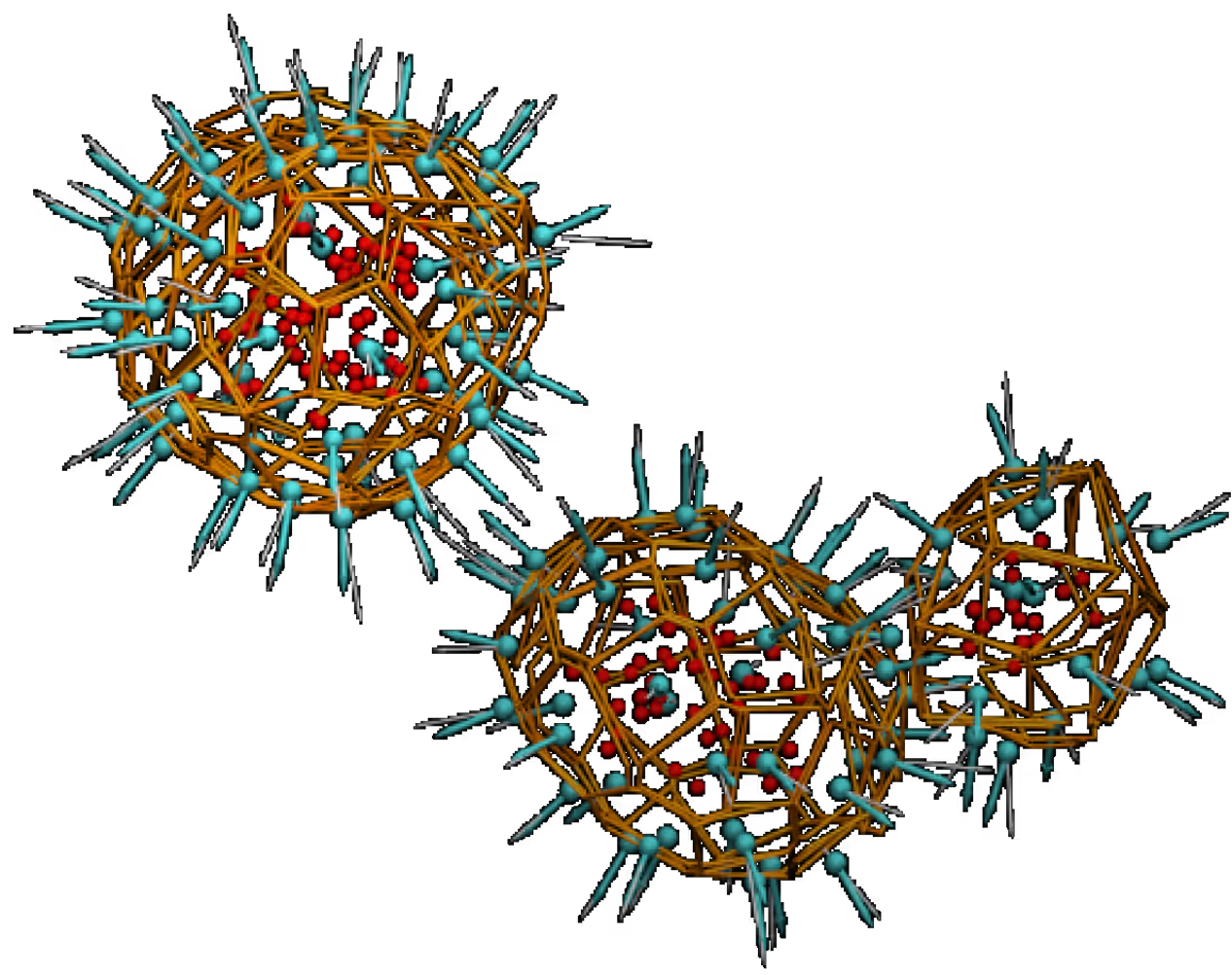
particle surface reconstructions (right) of the
nanoparticles with different molar ratio
of two studied compounds
04/ Nanoparticle Formulation Results
- The stable nanoparticles of desirable sizes are formed by the compound #1 or by the mixture of both with molar ratio up to 1:1.
- Higher ratios cause undesirable amorphous aggregates.
- These findings are successfully implemented in the
experimental optimization of nanoparticle formulation.