

Available from Reaxense
This protein is integrated into the Receptor.AI ecosystem as a prospective target with high therapeutic potential. We performed a comprehensive characterization of HLA class I histocompatibility antigen, alpha chain E including:
1. LLM-powered literature research
Our custom-tailored LLM extracted and formalized all relevant information about the protein from a large set of structured and unstructured data sources and stored it in the form of a Knowledge Graph. This comprehensive analysis allowed us to gain insight into HLA class I histocompatibility antigen, alpha chain E therapeutic significance, existing small molecule ligands, relevant off-targets, and protein-protein interactions.
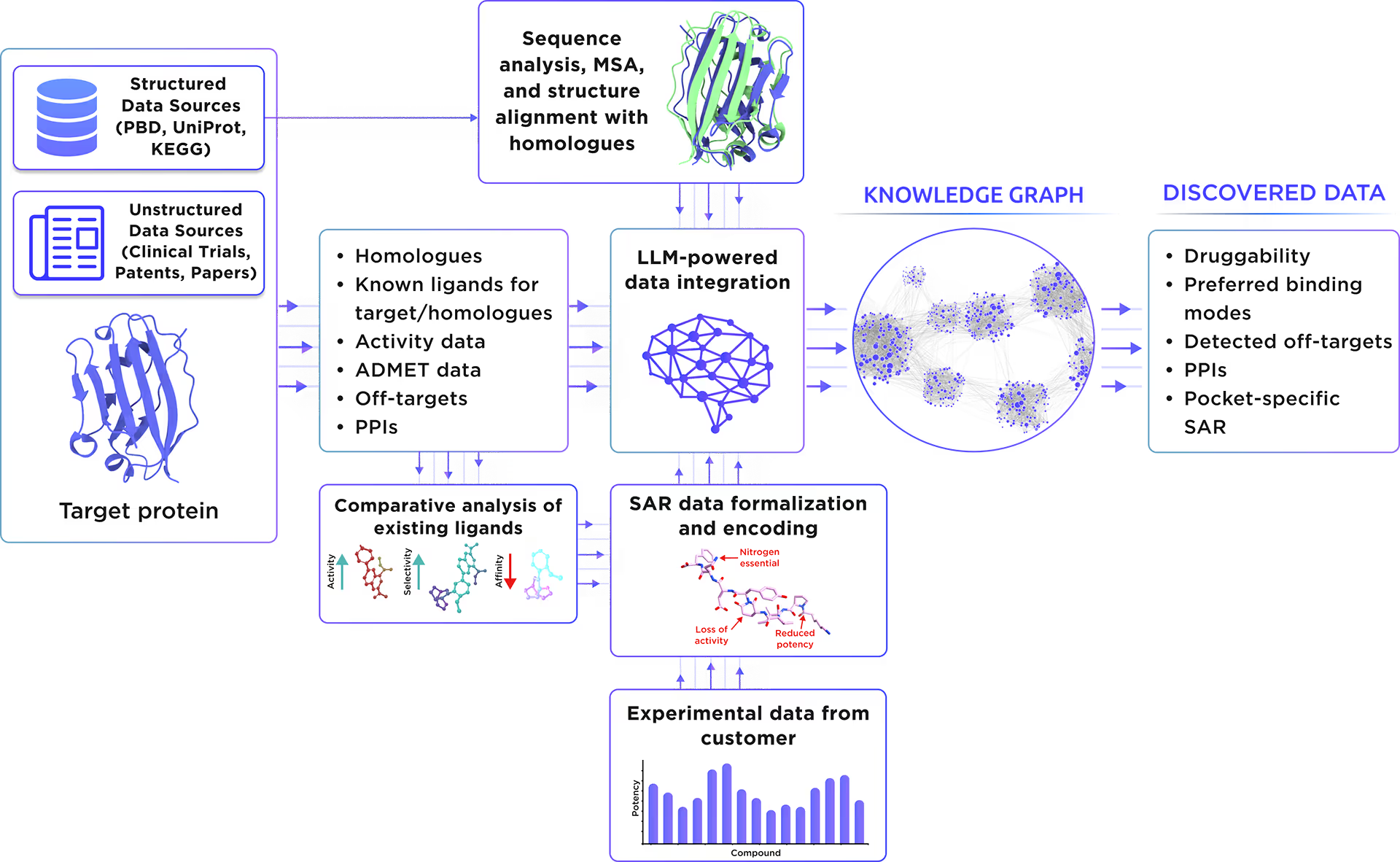
Fig. 1. Preliminary target research workflow
2. AI-Driven Conformational Ensemble Generation
Starting from the initial protein structure, we employed advanced AI algorithms to predict alternative functional states of HLA class I histocompatibility antigen, alpha chain E, including large-scale conformational changes along "soft" collective coordinates. Through molecular simulations with AI-enhanced sampling and trajectory clustering, we explored the broad conformational space of the protein and identified its representative structures. Utilizing diffusion-based AI models and active learning AutoML, we generated a statistically robust ensemble of equilibrium protein conformations that capture the receptor's full dynamic behavior, providing a robust foundation for accurate structure-based drug design.
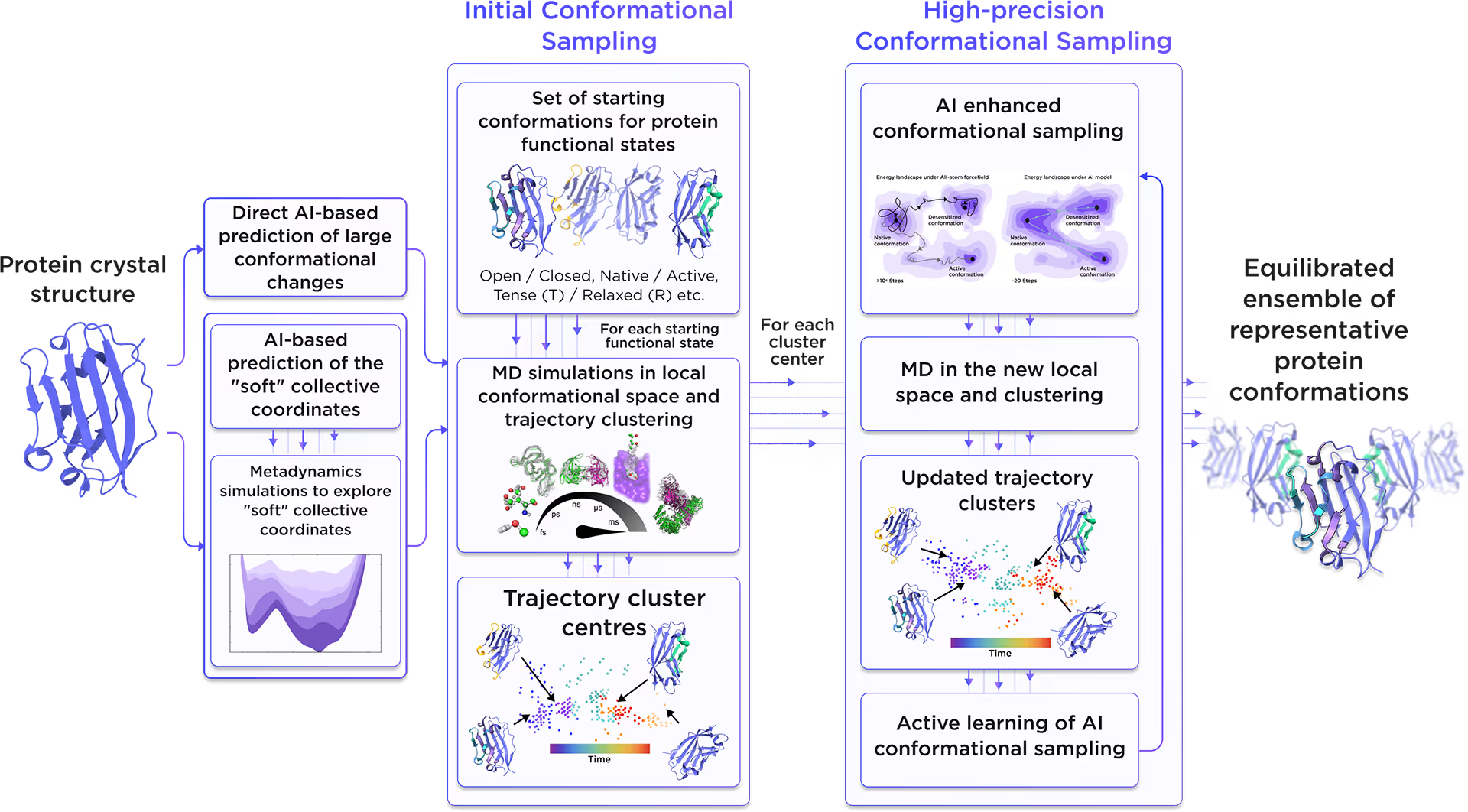
Fig. 2. AI-powered molecular dynamics simulations workflow
3. Binding pockets identification and characterization
We employed the AI-based pocket prediction module to discover orthosteric, allosteric, hidden, and cryptic binding pockets on the protein’s surface. Our technique integrates the LLM-driven literature search and structure-aware ensemble-based pocket detection algorithm that utilizes previously established protein dynamics. Tentative pockets are then subject to AI scoring and ranking with simultaneous detection of false positives. In the final step, the AI model assesses the druggability of each pocket enabling a comprehensive selection of the most promising pockets for further targeting.
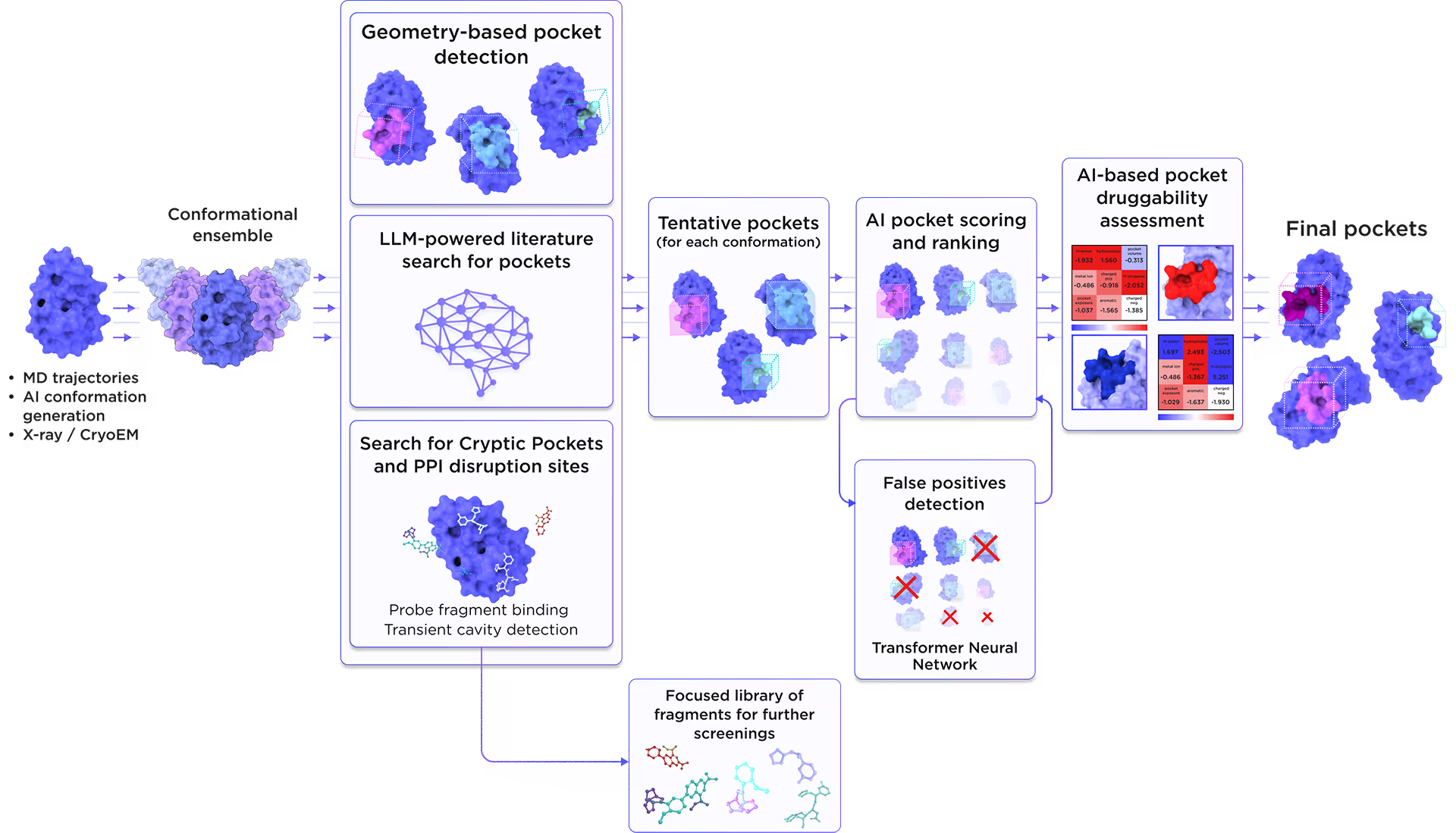
Fig. 3. AI-based binding pocket detection workflow
4. AI-Powered Virtual Screening
Our ecosystem is equipped to perform AI-driven virtual screening on HLA class I histocompatibility antigen, alpha chain E. With access to a vast chemical space and cutting-edge AI docking algorithms, we can rapidly and reliably predict the most promising, novel, diverse, potent, and safe small molecule ligands of HLA class I histocompatibility antigen, alpha chain E. This approach allows us to achieve an excellent hit rate and to identify compounds ready for advanced lead discovery and optimization.
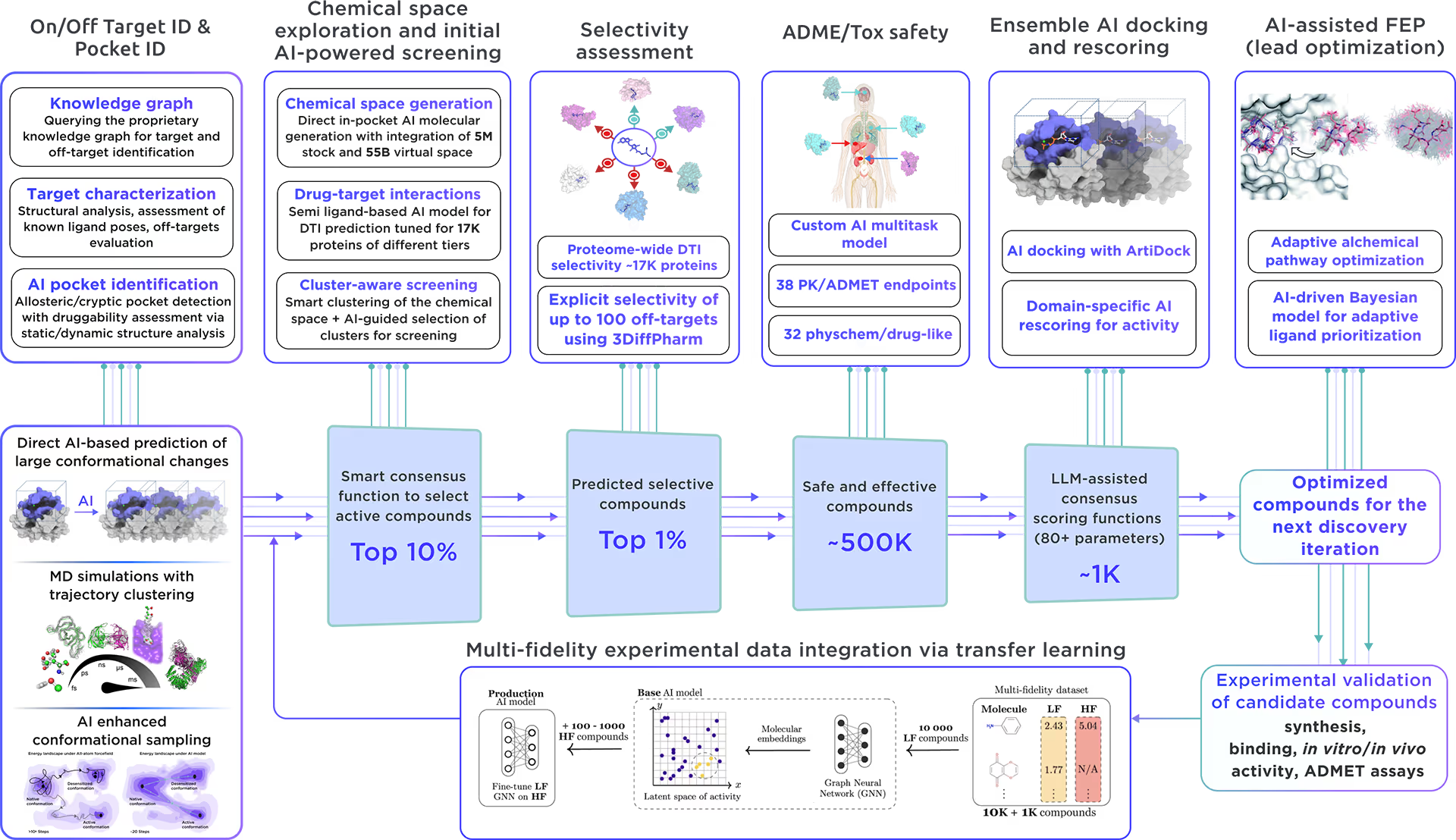
Fig. 4. The screening workflow of Receptor.AI
Receptor.AI, in partnership with Reaxense, developed a next-generation technology for on-demand focused library design to enable extensive target exploration.
The focused library for HLA class I histocompatibility antigen, alpha chain E includes a list of the most effective modulators, each annotated with 38 ADME-Tox and 32 physicochemical and drug-likeness parameters. Furthermore, each compound is shown with its optimal docking poses, affinity scores, and activity scores, offering a detailed summary.
HLA class I histocompatibility antigen, alpha chain E
partner:
Reaxense
upacc:
P13747
UPID:
HLAE_HUMAN
Alternative names:
MHC class I antigen E
Alternative UPACC:
P13747; E2G051; Q30169; Q6DU44; Q9BT83; Q9GIY7; Q9GIY8
Background:
HLA class I histocompatibility antigen, alpha chain E (HLA-E), a non-classical major histocompatibility class Ib molecule, plays a crucial role in immune self-nonself discrimination. It forms a complex with B2M/beta-2-microglobulin to bind self-peptides from classical MHC class Ia molecules, functioning as a ligand for NK cell inhibitory receptors, thus enabling NK cells to tolerate self. HLA-E's interaction with peptides from stress-induced chaperones or viral proteins alters its recognition by NK cells, impacting immune response.
Therapeutic significance:
Understanding the role of HLA-E could open doors to potential therapeutic strategies, especially considering its involvement in immune evasion mechanisms of pathogens like HIV-1, human cytomegalovirus, and SARS-CoV-2. Its ability to modulate NK cell activity offers a promising avenue for enhancing antiviral and antitumor immunity.