8M
stock library
screened
80%
psoriasis severity
reduction
Safe
No immunosuppression observed in vivo
Multi-indication for multiple autoimmune diseases
01/ Background
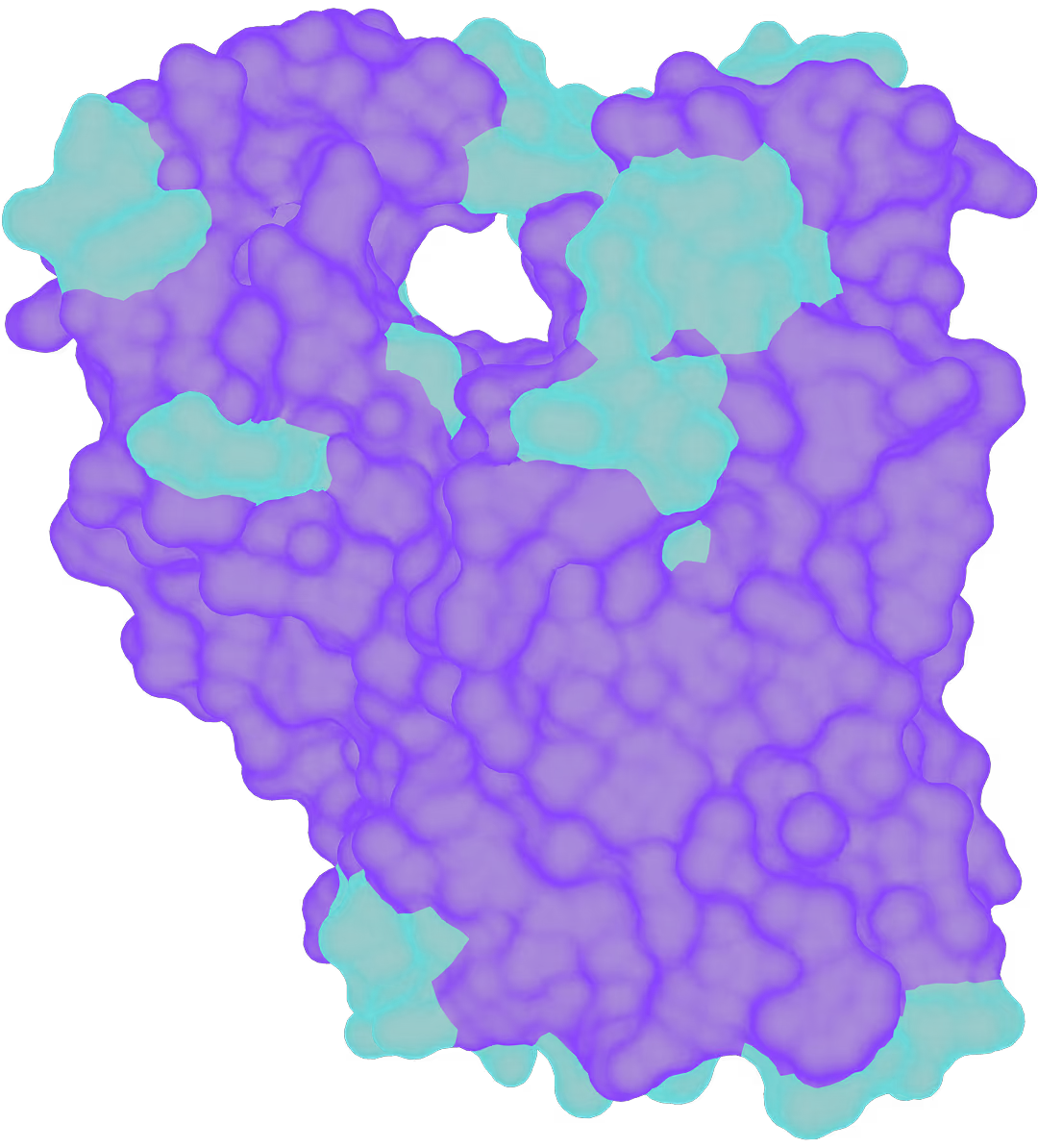
- The target is a membrane transporter, mediator in autoimmune signaling pathway (colitis, dermatitis, psoriasis and other diseases).
- The goal is to design a novel small molecule inhibitor which surpasses competitors in efficacy and safety.
02/ Methodology
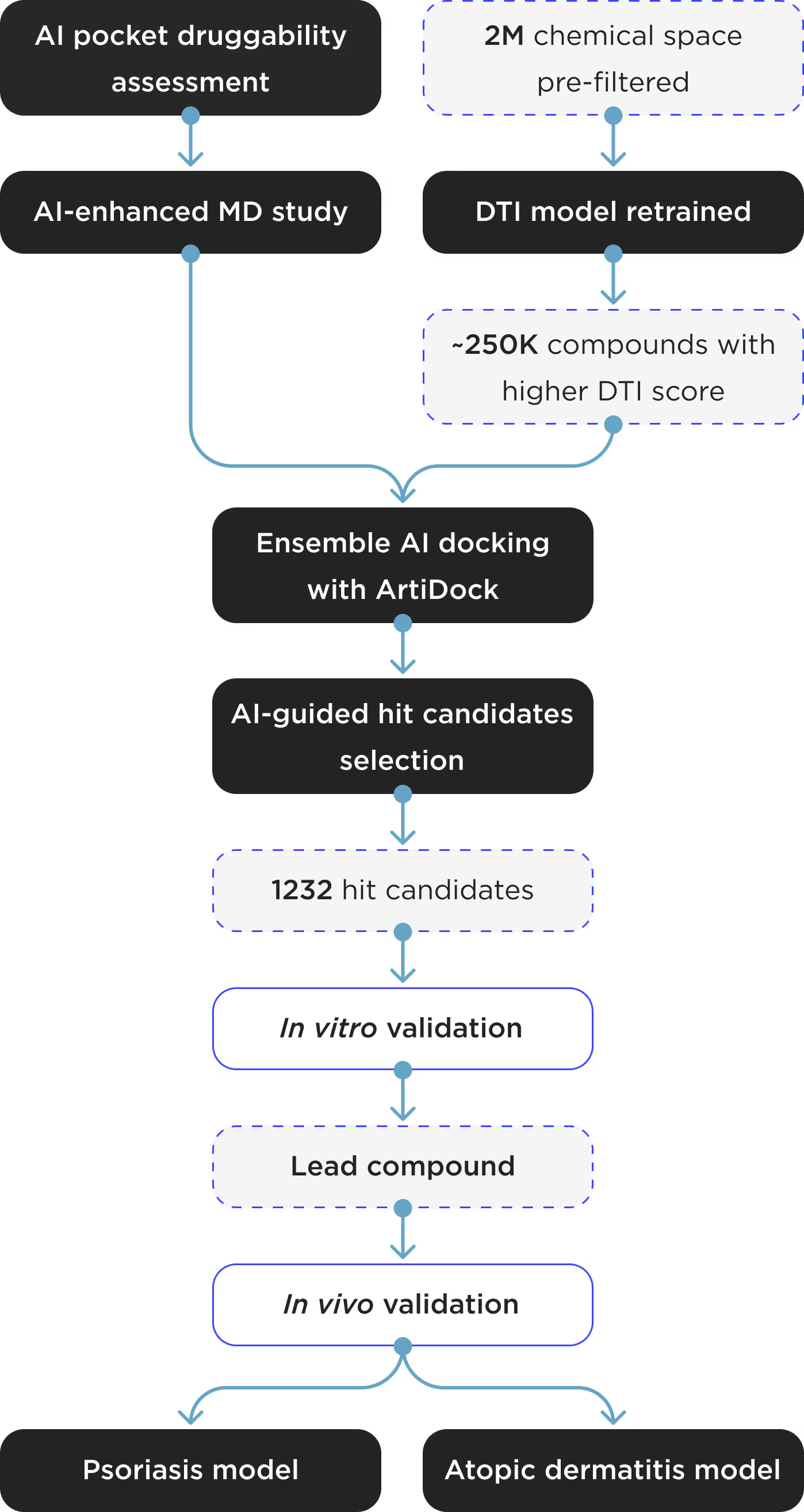
- AI druggability assessment of binding pockets.
- Extensive AI-enhanced MD.
- 2M chemical space pre-filtered on ADMET and drug likeness from 8M stock library.
- Proprietary AI DTI model retrained on known inhibitors.
- ~250K compounds with higher DTI score subject to ensemble AI docking with ArtiDock.
- ~17K ligands chosen for AI-guided hit candidates selection.
- 1232 hit candidates subject to in vitro validation.
- The lead compound subject to in vivo validation.
03/ Results
- Lead shows higher efficacy than competitors.
- In psoriasis model shown improved effect with topical application.
- Earlier impact due to upstream target.
- Lead compound proved effective in colitis, atopic dermatitis, and psoriasis in vivo models.
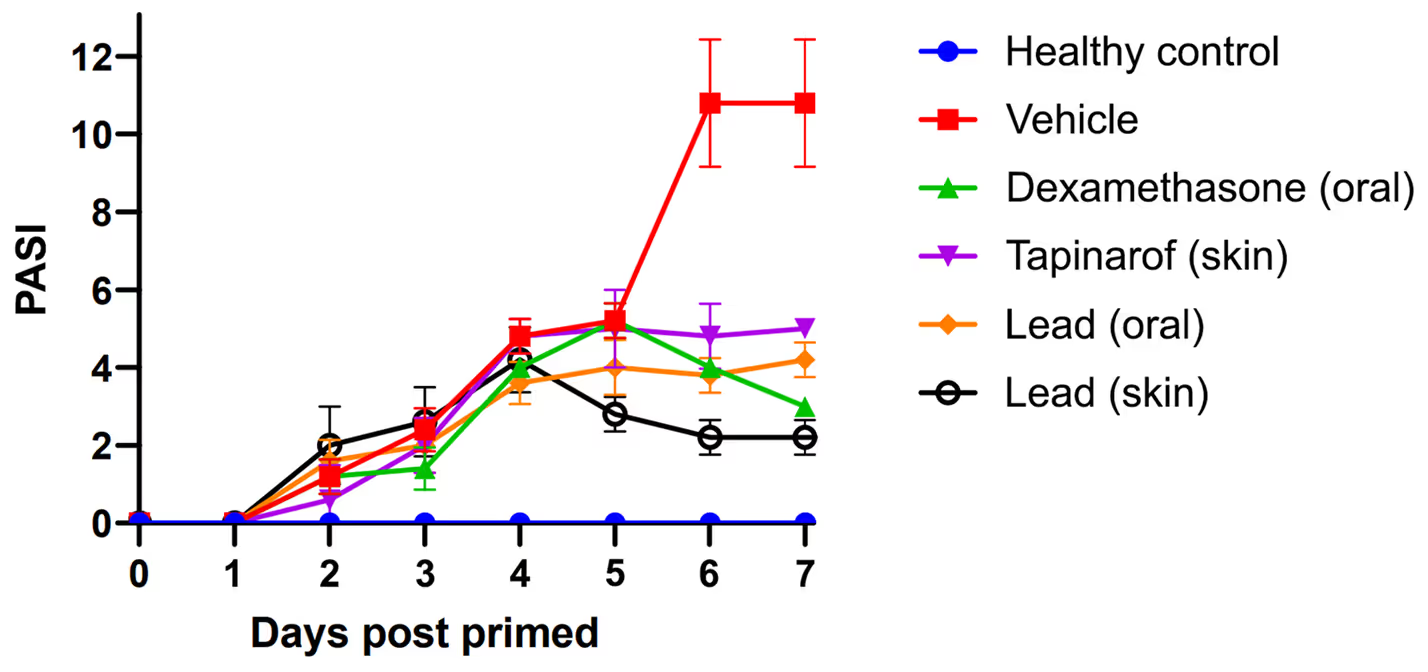
*Psoriasis Area and Severity Index (PASI) in
psoriasis model for 7 days of treatment
04/ Safety and accessibility
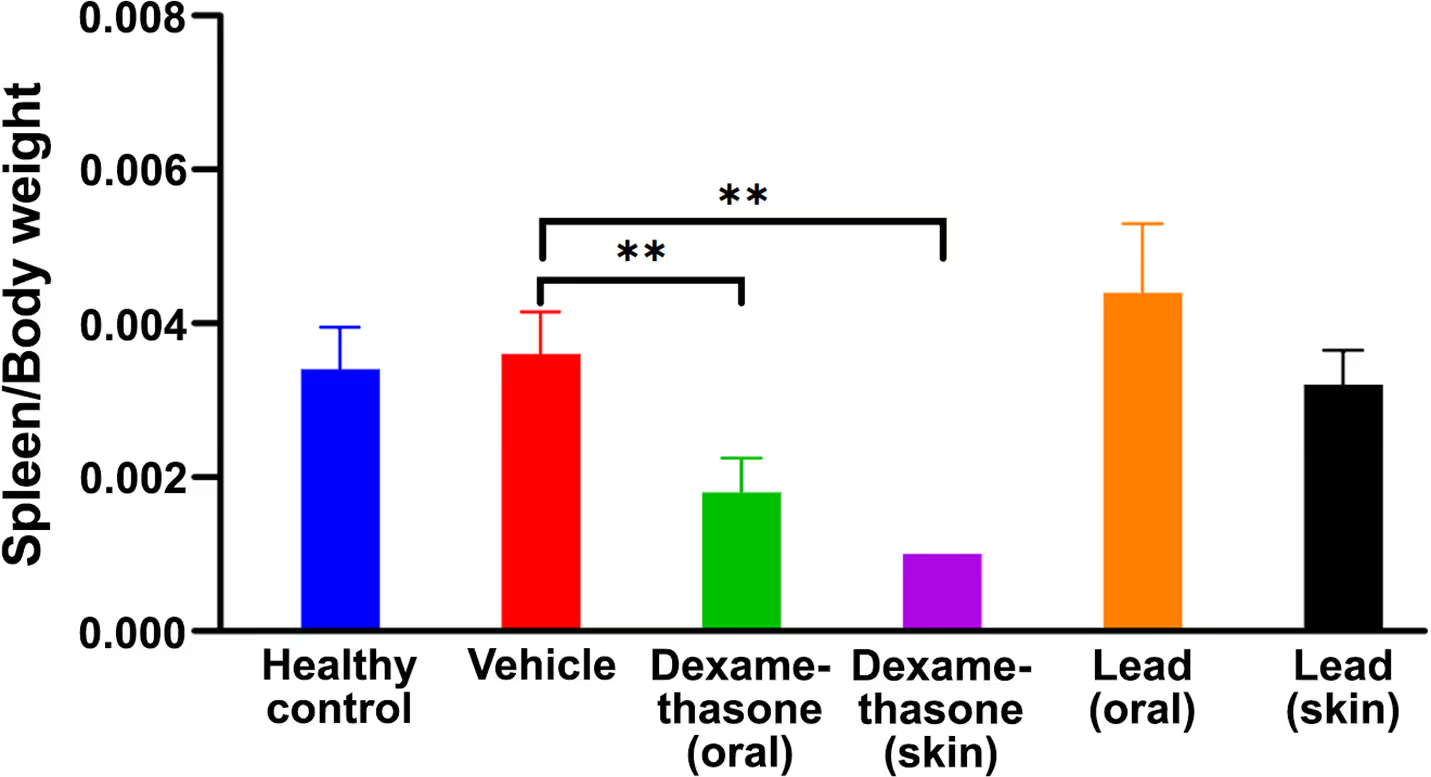
- No immunosuppressive effect was observed in vivo opposed to competitor.
- Daily oral use was validated, topical use is also possible.
- Better safety and accessibility enable use in prolonged treatment.