The PEPTOR™ Platform for Modern Peptide Design
Announcement



Full Text
Peptide therapeutics continue to expand their presence across metabolic disease, oncology, and inflammation. Their ability to engage extended or conformationally complex targets – including many that small molecules or antibodies struggle to reach – makes them a valuable modality in contemporary drug discovery.
However, the field still faces a persistent bottleneck. Discovery technologies readily deliver high-affinity sequences, but only a small fraction achieve the permeability, stability, and pharmacokinetic behavior required of a viable drug candidate. The challenge is no longer identifying active peptides; it is engineering drug-like ones.
This gap is the motivation behind PEPTOR™, Receptor.AI’s platform for peptide design.
Limitations of traditional peptide optimization
Most peptide workflows remain structurally linear. After an initial binder is obtained (via display technologies or rational derivation), optimization typically proceeds property by property – improving potency, then stabilizing the scaffold, then attempting to address permeability or PK. These property tracks often interfere with one another, and single-parameter adjustments commonly introduce new liabilities.
Examples are well known:
- Cyclization improves protease resistance but may compromise solubility.
- N-methylation can enhance passive diffusion while disrupting backbone geometry.
- Minor side-chain changes can alter conformational equilibria in unpredictable ways.
This fragmentation explains why many promising sequences fail to progress beyond early optimization.
A more integrated approach to peptide engineering
Three developments now make a unified design strategy possible:
1. A broader chemical repertoire
D-amino acids, β/γ-residues, N-methyl groups, and non-peptidic linkers allow fine control over rigidity, polarity, and resistance to enzymatic degradation. In peptide chemistry, drug-likeness increasingly depends on tailored function rather than adherence to classical small-molecule rules.
2. Physics- and ML-based modeling for guidance
Molecular dynamics and machine-learning models provide insight into folding behavior, solvent exposure, and relative permeability trends. While not a substitute for experimental assays, these methods help prioritize which design directions are most likely to yield tractable candidates.
3. Integrated, multi-objective optimization frameworks
This is the domain in which PEPTOR™ operates.
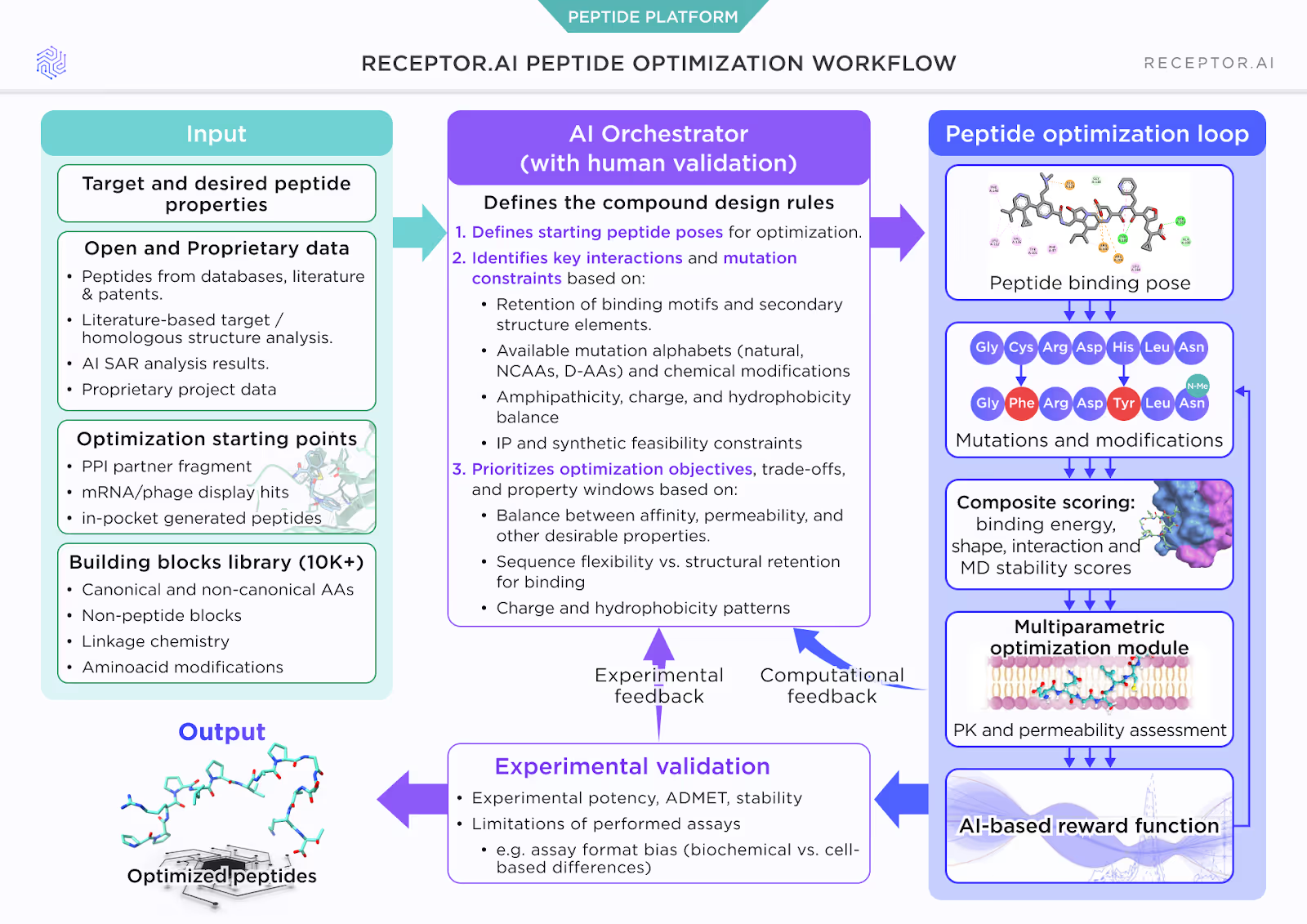
How PEPTOR™ enables multi-parameter peptide design
PEPTOR™ combines chemistry, modeling, and experimental data into a single coordinated workflow.
Coordinated orchestration
An AI layer manages the design process: defining allowed modifications, ensuring structural constraints are respected, and maintaining consistency across iterations.
Optimization loop as the design engine
The core design work is performed by an optimization loop that generates peptide variants and evaluates them across multiple criteria simultaneously, including:
- structural retention and pharmacophore fidelity
- permeability property windows
- conformational stability
- predicted potency and ADMET liabilities
This approach avoids the sequential, property-by-property adjustments that often derail classical peptide SAR.
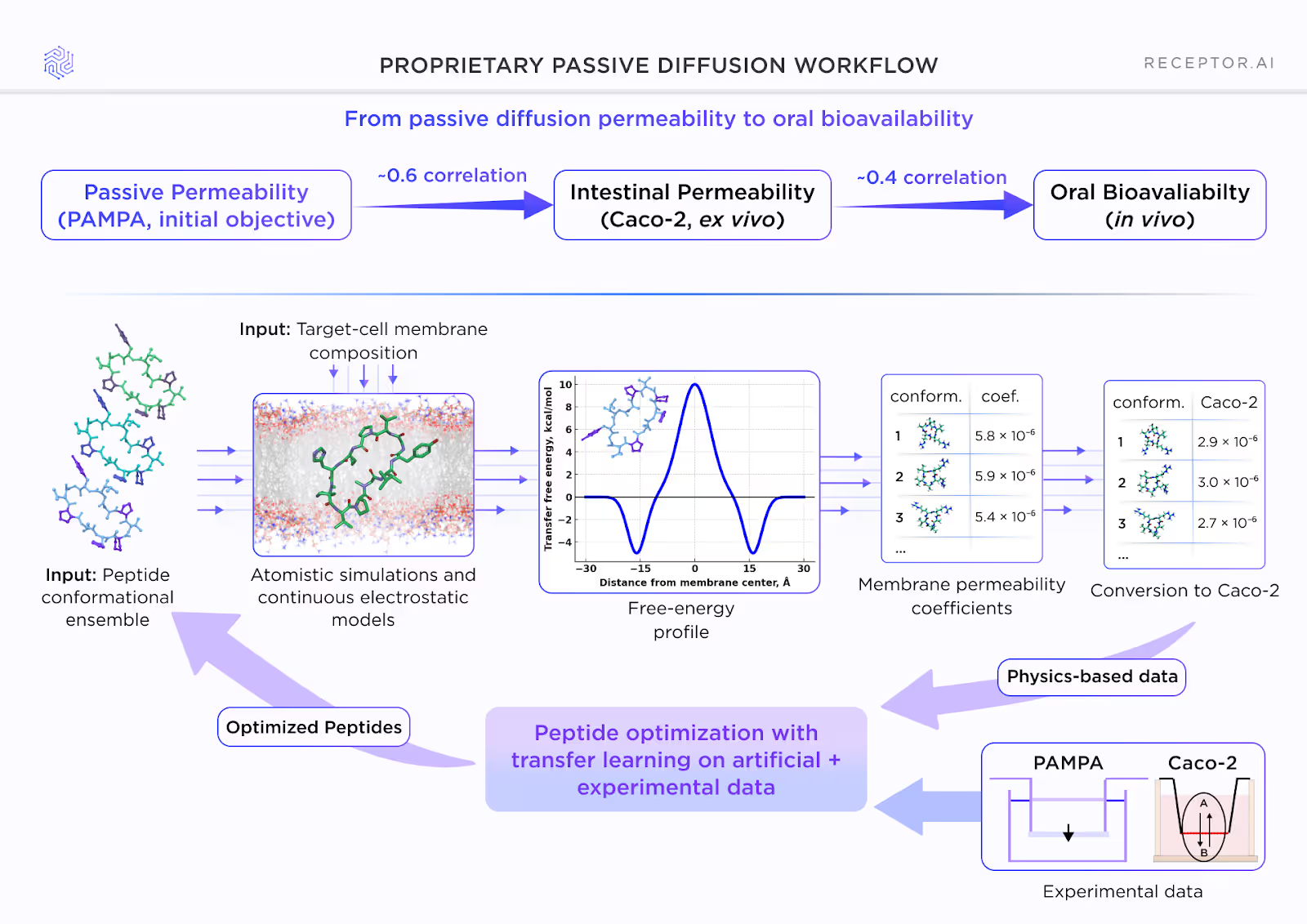
Experiment-anchored permeability modeling
Receptor.AI’s permeability optimization is grounded in a workflow that links physics-based atomistic simulations with experimental readouts. The workflow begins by generating a conformational ensemble of each peptide and simulating its interaction with a target-cell membrane composition. These simulations yield transfer free-energy profiles, which are converted into membrane permeability coefficients and then converted into Caco-2–equivalent values. These and experimental PAMPA and Caco-2 measurements are incorporated through transfer learning, allowing the system to refine its predictions across optimization cycles.
Toward practical peptide medicinal chemistry
The emergence of expanded chemical space, improved modeling, and integrated optimization has shifted peptide design toward a more systematic discipline. PEPTOR™ was developed to support this transition: from individual property adjustments to coherent, multi-objective engineering.
Ultimately, the peptides most likely to succeed clinically are not merely strong binders, but those deliberately designed for defined conformation, sufficient exposure, and reliable developability. Our goal with PEPTOR™ is to make this type of design both routine and scalable.

