

Available from Reaxense
This protein is integrated into the Receptor.AI ecosystem as a prospective target with high therapeutic potential. We performed a comprehensive characterization of Heterogeneous nuclear ribonucleoprotein A1 including:
1. LLM-powered literature research
Our custom-tailored LLM extracted and formalized all relevant information about the protein from a large set of structured and unstructured data sources and stored it in the form of a Knowledge Graph. This comprehensive analysis allowed us to gain insight into Heterogeneous nuclear ribonucleoprotein A1 therapeutic significance, existing small molecule ligands, relevant off-targets, and protein-protein interactions.
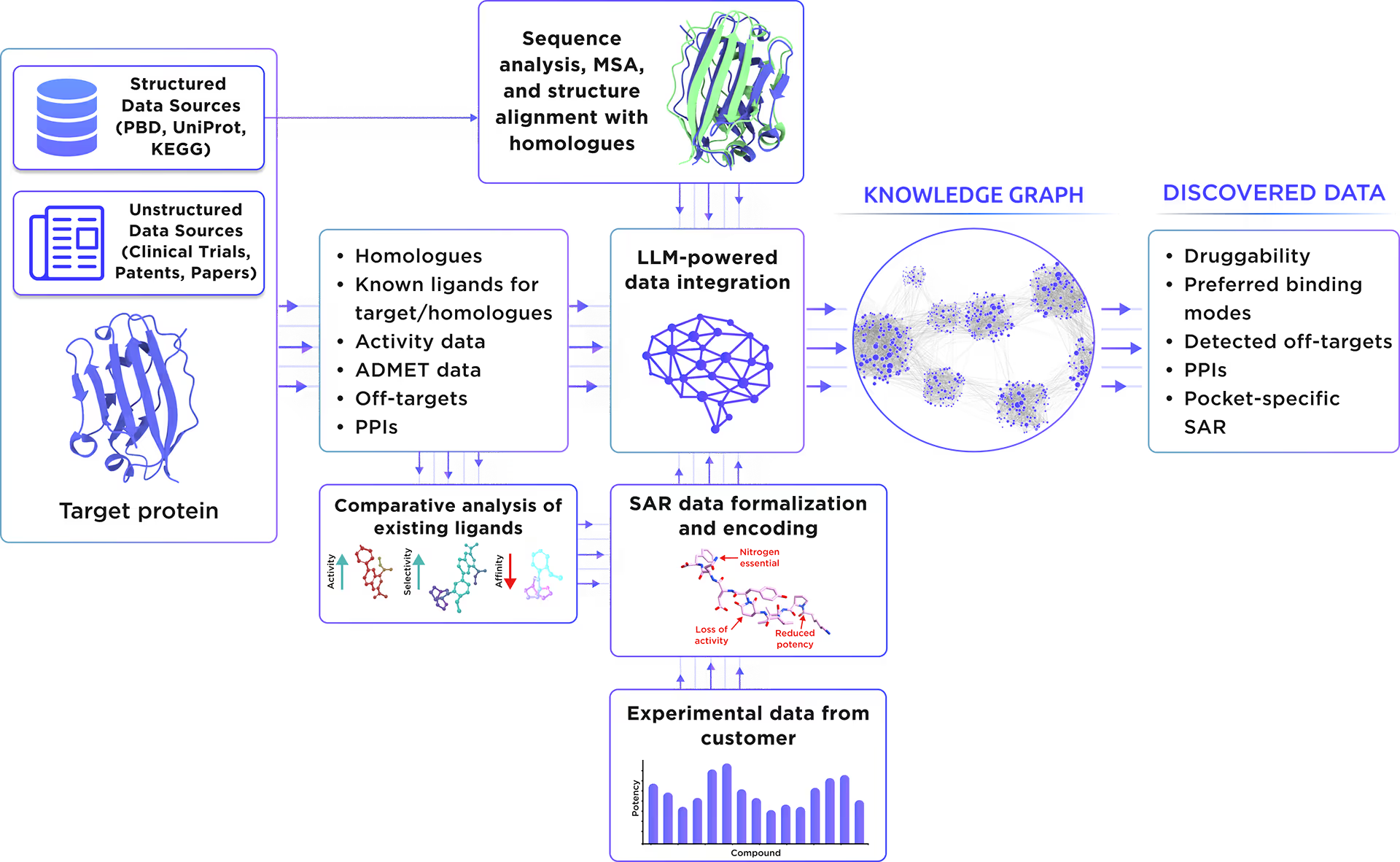
Fig. 1. Preliminary target research workflow
2. AI-Driven Conformational Ensemble Generation
Starting from the initial protein structure, we employed advanced AI algorithms to predict alternative functional states of Heterogeneous nuclear ribonucleoprotein A1, including large-scale conformational changes along "soft" collective coordinates. Through molecular simulations with AI-enhanced sampling and trajectory clustering, we explored the broad conformational space of the protein and identified its representative structures. Utilizing diffusion-based AI models and active learning AutoML, we generated a statistically robust ensemble of equilibrium protein conformations that capture the receptor's full dynamic behavior, providing a robust foundation for accurate structure-based drug design.
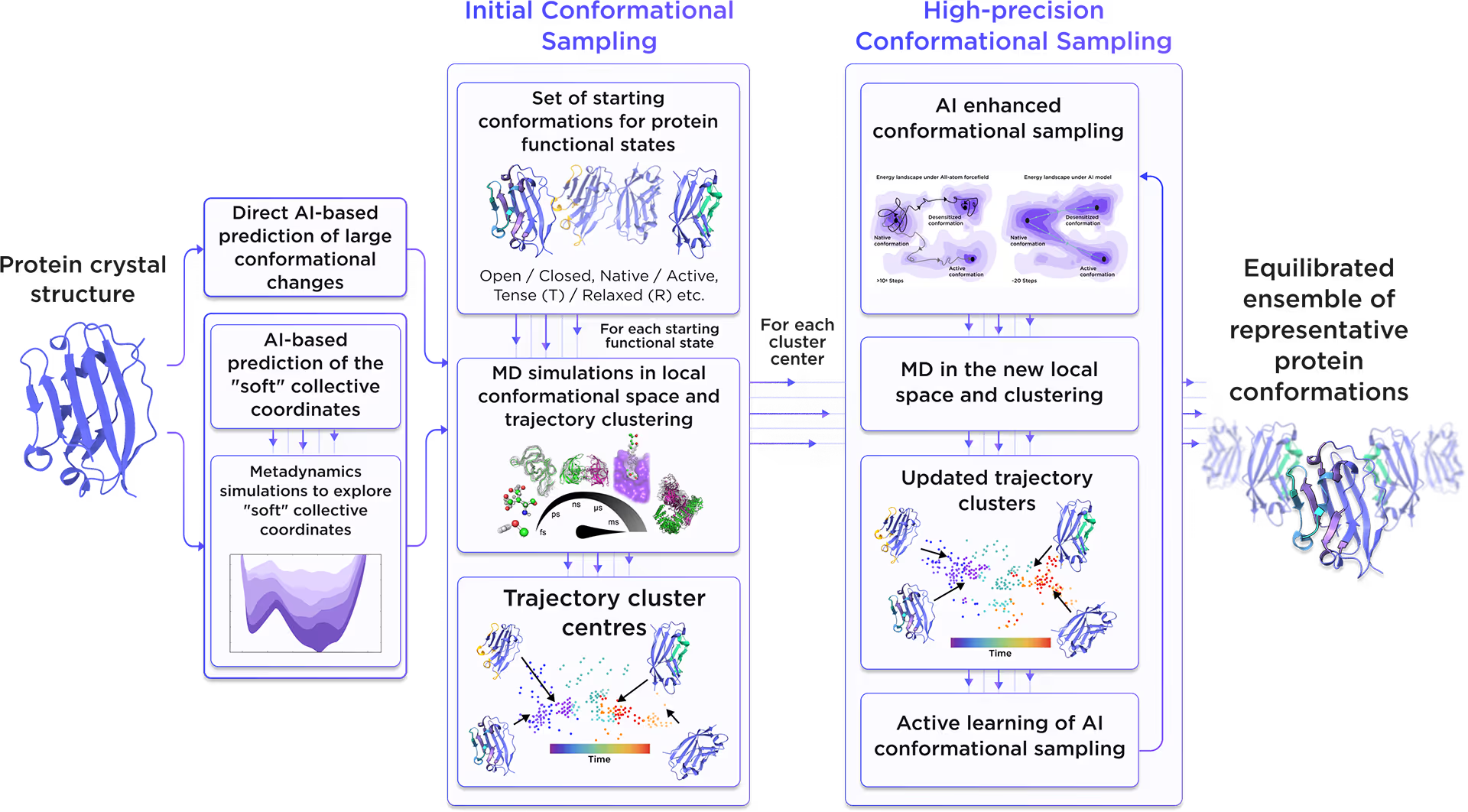
Fig. 2. AI-powered molecular dynamics simulations workflow
3. Binding pockets identification and characterization
We employed the AI-based pocket prediction module to discover orthosteric, allosteric, hidden, and cryptic binding pockets on the protein’s surface. Our technique integrates the LLM-driven literature search and structure-aware ensemble-based pocket detection algorithm that utilizes previously established protein dynamics. Tentative pockets are then subject to AI scoring and ranking with simultaneous detection of false positives. In the final step, the AI model assesses the druggability of each pocket enabling a comprehensive selection of the most promising pockets for further targeting.
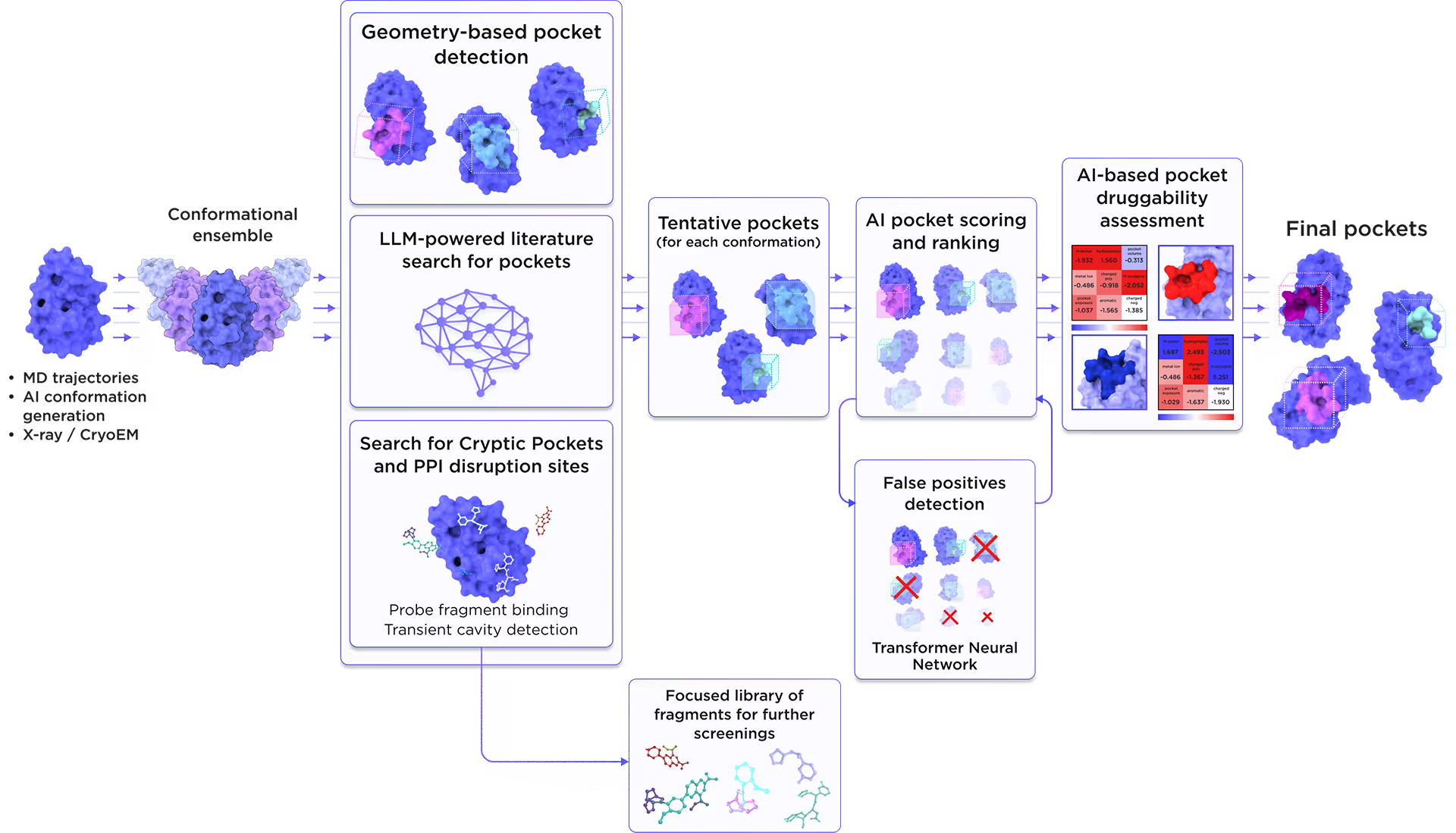
Fig. 3. AI-based binding pocket detection workflow
4. AI-Powered Virtual Screening
Our ecosystem is equipped to perform AI-driven virtual screening on Heterogeneous nuclear ribonucleoprotein A1. With access to a vast chemical space and cutting-edge AI docking algorithms, we can rapidly and reliably predict the most promising, novel, diverse, potent, and safe small molecule ligands of Heterogeneous nuclear ribonucleoprotein A1. This approach allows us to achieve an excellent hit rate and to identify compounds ready for advanced lead discovery and optimization.
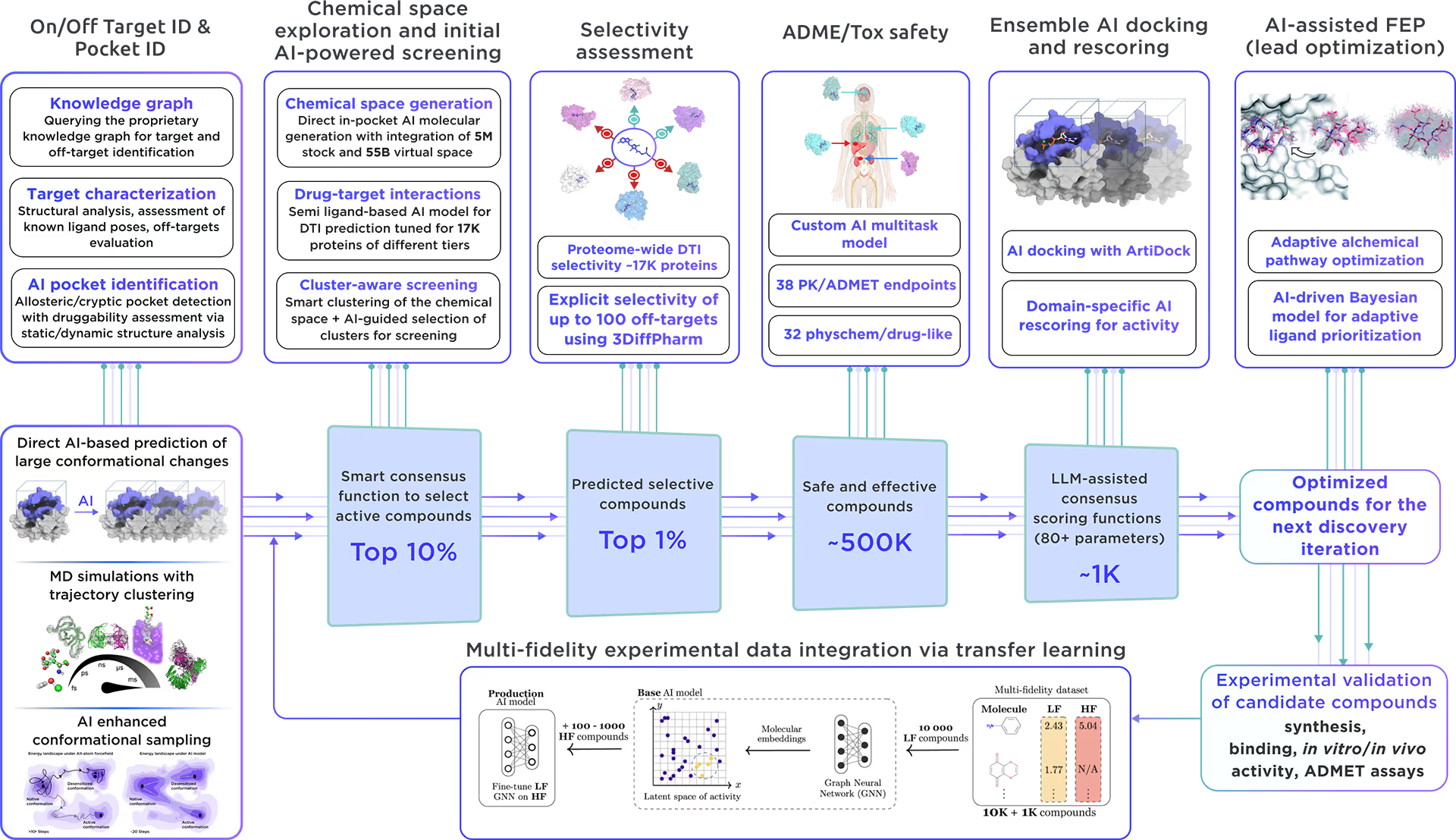
Fig. 4. The screening workflow of Receptor.AI
Receptor.AI, in partnership with Reaxense, developed a next-generation technology for on-demand focused library design to enable extensive target exploration.
The focused library for Heterogeneous nuclear ribonucleoprotein A1 includes a list of the most effective modulators, each annotated with 38 ADME-Tox and 32 physicochemical and drug-likeness parameters. Furthermore, each compound is shown with its optimal docking poses, affinity scores, and activity scores, offering a detailed summary.
Heterogeneous nuclear ribonucleoprotein A1
partner:
Reaxense
upacc:
P09651
UPID:
ROA1_HUMAN
Alternative names:
Helix-destabilizing protein; Single-strand RNA-binding protein; hnRNP core protein A1
Alternative UPACC:
P09651; A8K4Z8; Q3MIB7; Q6PJZ7
Background:
Heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) plays a pivotal role in cellular processes, including pre-mRNA packaging, mRNA transport, and splice site selection. It influences the splicing of pyruvate kinase PKM and inhibits the translation of APAF1, impacting apoptosis. Additionally, hnRNP A1 interacts with miRNA hairpins and is implicated in microbial infections, highlighting its versatility in RNA biology.
Therapeutic significance:
hnRNP A1's involvement in diseases such as Inclusion body myopathy with early-onset Paget disease and Amyotrophic lateral sclerosis 20 underscores its potential as a therapeutic target. Understanding its role could open doors to novel treatments for these debilitating conditions.